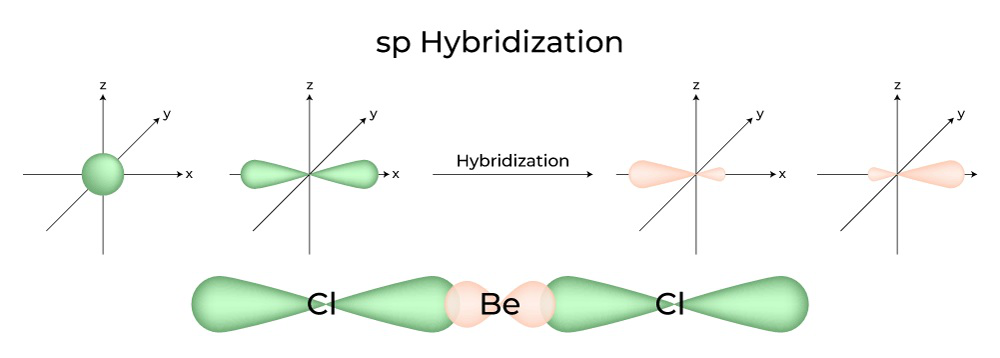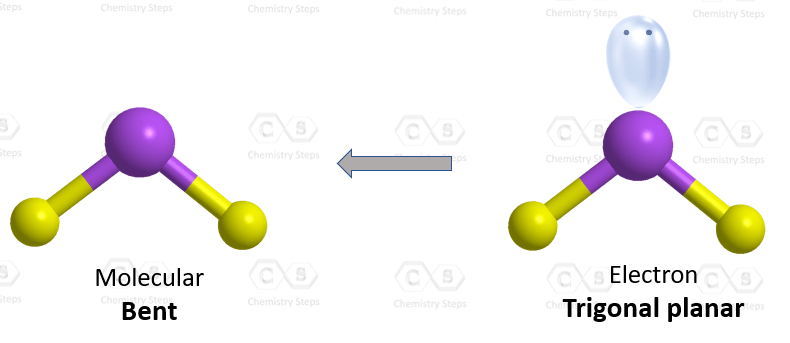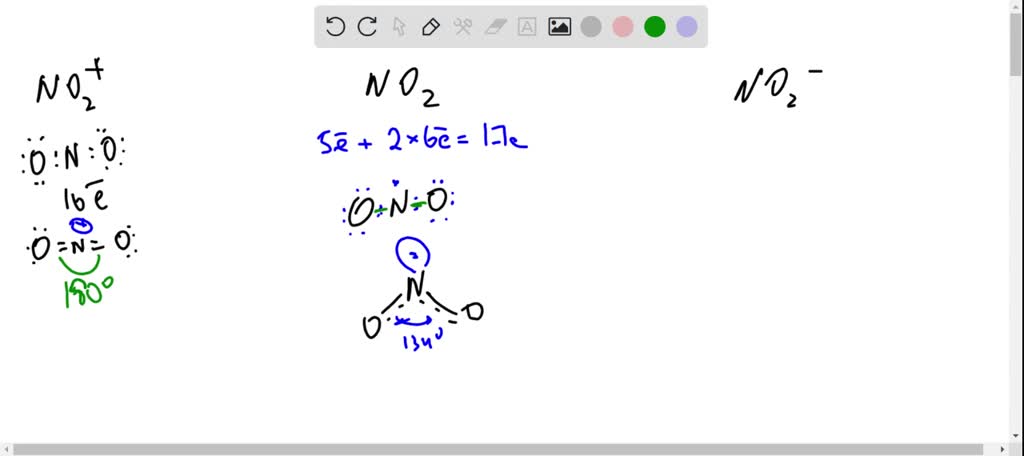
SOLVED: The three species NO2+, NO2 and NO2- all have a central N atom. The O-N-O bond angles in the three species are 180, 134 and 115 respectively. Explain this variation in

inorganic chemistry - Hybridization of orbitals and forming of bonds in the nitrogen dioxide molecule - Chemistry Stack Exchange

Bond Angle And Hybridization Of NO2,NO2-,NO2+,NO3-||Lewis Dot Structure||iit,neet,cbse, icse,kvpy|| - YouTube

Classification of Negative Charge Discriminate Hybridization with Aromatic and Anti-aromatic Behavior of Organic Compounds - Innovative Mnemonics

The hybridization of atomic orbitals of nitrogen is `NO_(2)^(+), NO_(3)^(-)`, and `NH_(4)^(+)` respe - YouTube

NO2- , NO2, NO2+ Lewis dot structure, Identification of Co-ordinate Bond and hybridisation - YouTube
![The types of hybrid orbitals of nitrogen in \\[NO_{2}^{+}\\], \\[NO_{3}^{-}\\] and \\[NH_{4}^{+}\\] respectively are expected to be:A. \\[sp,\\text{ }s{{p}^{3}}\\text{ }and\\text{ }s{{p}^{2}}\\]B. \\[sp,\\text{ }s{{p}^{2}}\\text{ }and\\text{ }s{{p}^{3 ... The types of hybrid orbitals of nitrogen in \\[NO_{2}^{+}\\], \\[NO_{3}^{-}\\] and \\[NH_{4}^{+}\\] respectively are expected to be:A. \\[sp,\\text{ }s{{p}^{3}}\\text{ }and\\text{ }s{{p}^{2}}\\]B. \\[sp,\\text{ }s{{p}^{2}}\\text{ }and\\text{ }s{{p}^{3 ...](https://www.vedantu.com/question-sets/34556bfa-0533-4f6a-a822-b7426c5f37ea4031147211903642416.png)