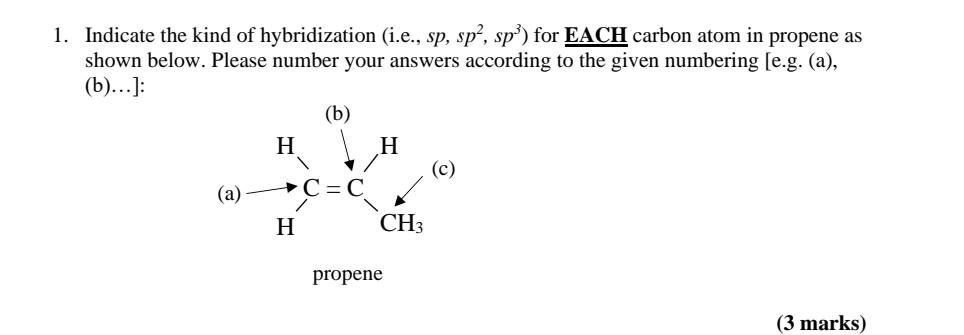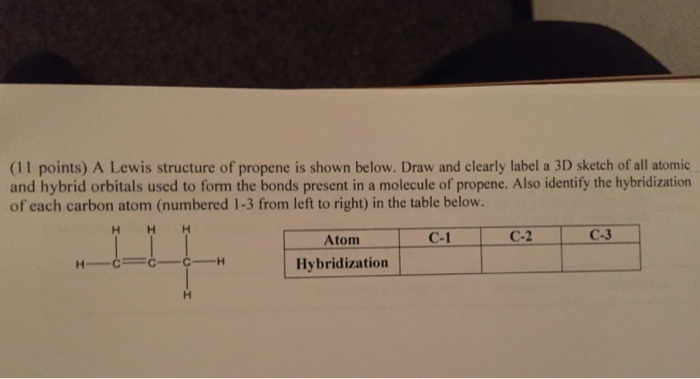
SOLVED: 21 What is the hybridization for each carbon in propene? Carbon on the lefi Carbon in the center Carbon on the right C=C–H H Propene 22 What is the hybridization for

How exactly is this carbon considered sp2 hybridized when it has 3 substituents and a lone pair? : r/OrganicChemistry
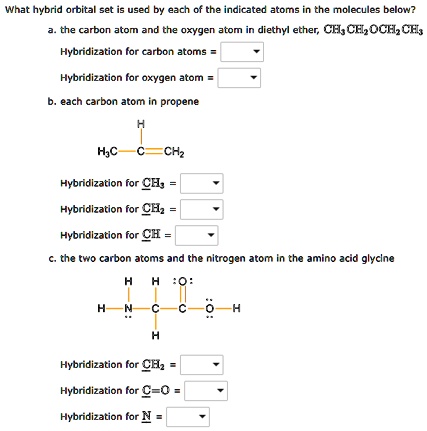
SOLVED: Text: What hybrid orbital set is used by each of the indicated atoms in the molecules below? a. the carbon atom and the oxygen atom in diethyl ether, CH3COCH2CH3 Hybridization for
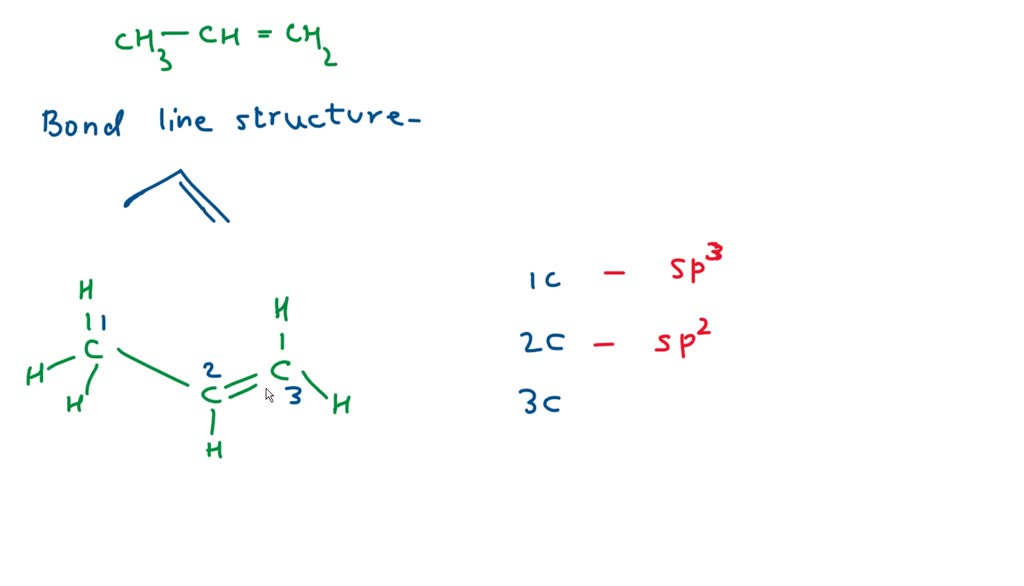
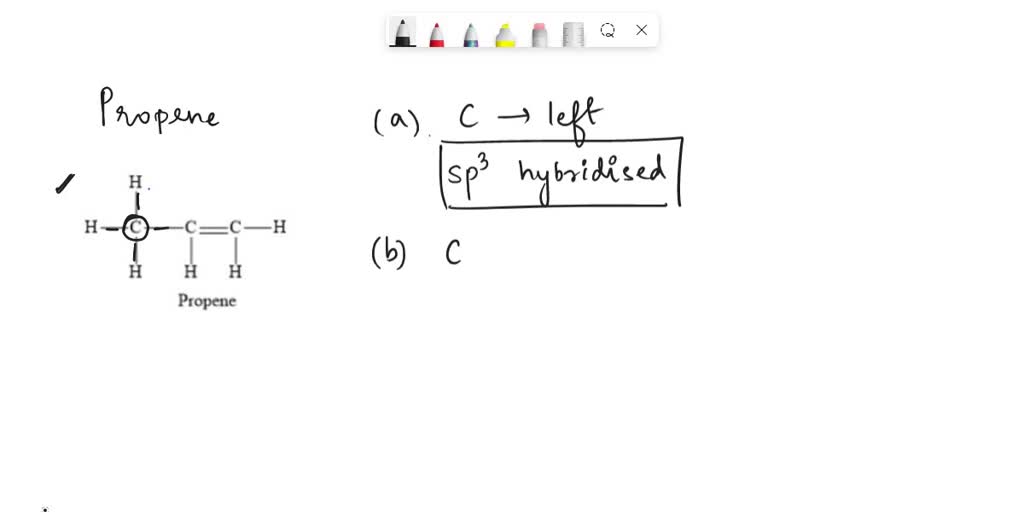
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...
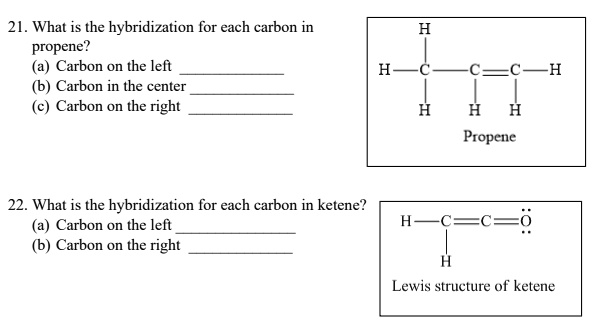
SOLVED: 21 What is the hybridization for each carbon in propene? Carbon on the lefi Carbon in the center Carbon on the right C=C–H H Propene 22 What is the hybridization for
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...

Orbital Hybridization Practice Problems - Organic Chemistry #stemeducation #stem #chemistry - YouTube
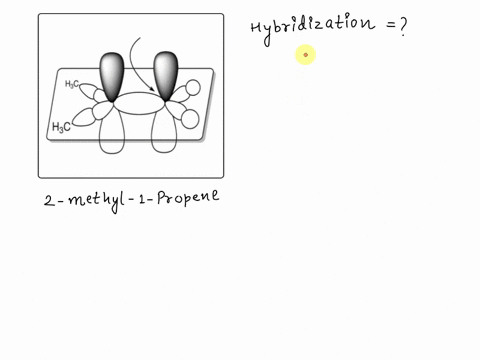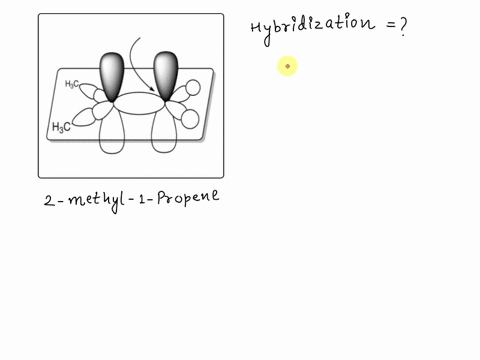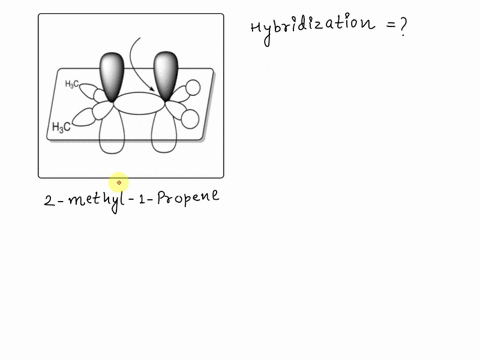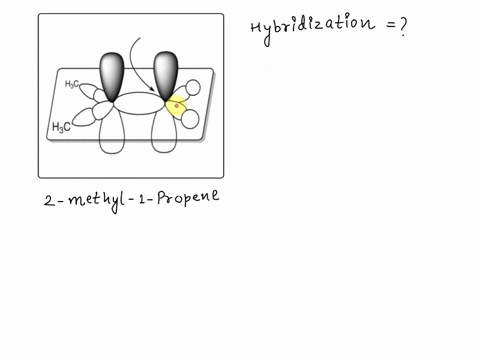
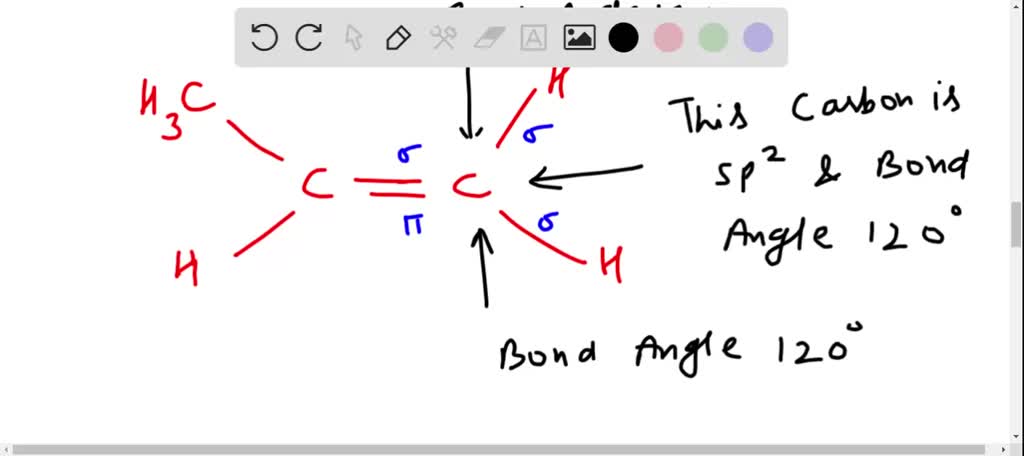
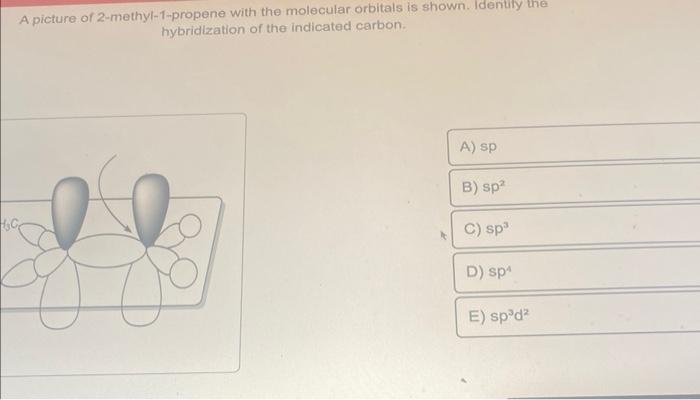
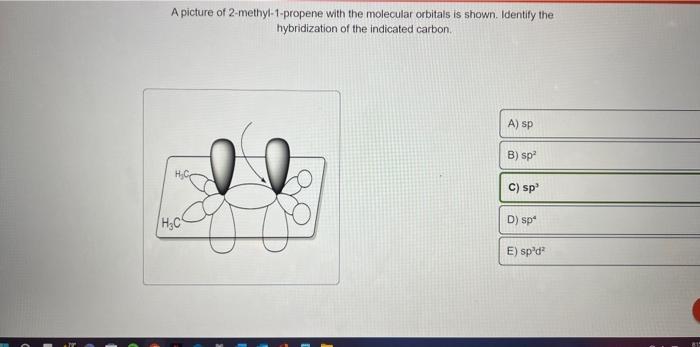
SOLVED: Texts: A picture of 2-methyl-1-propene with the molecular orbitals is shown. Identify the hybridization of the indicated carbon. A) sp B) sp2 C) sp D) sp3 E) sp2
How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora

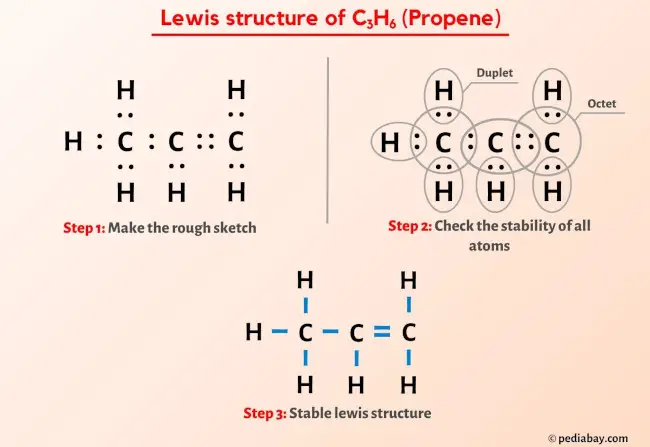
Propene C3H6: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure – infographic
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...