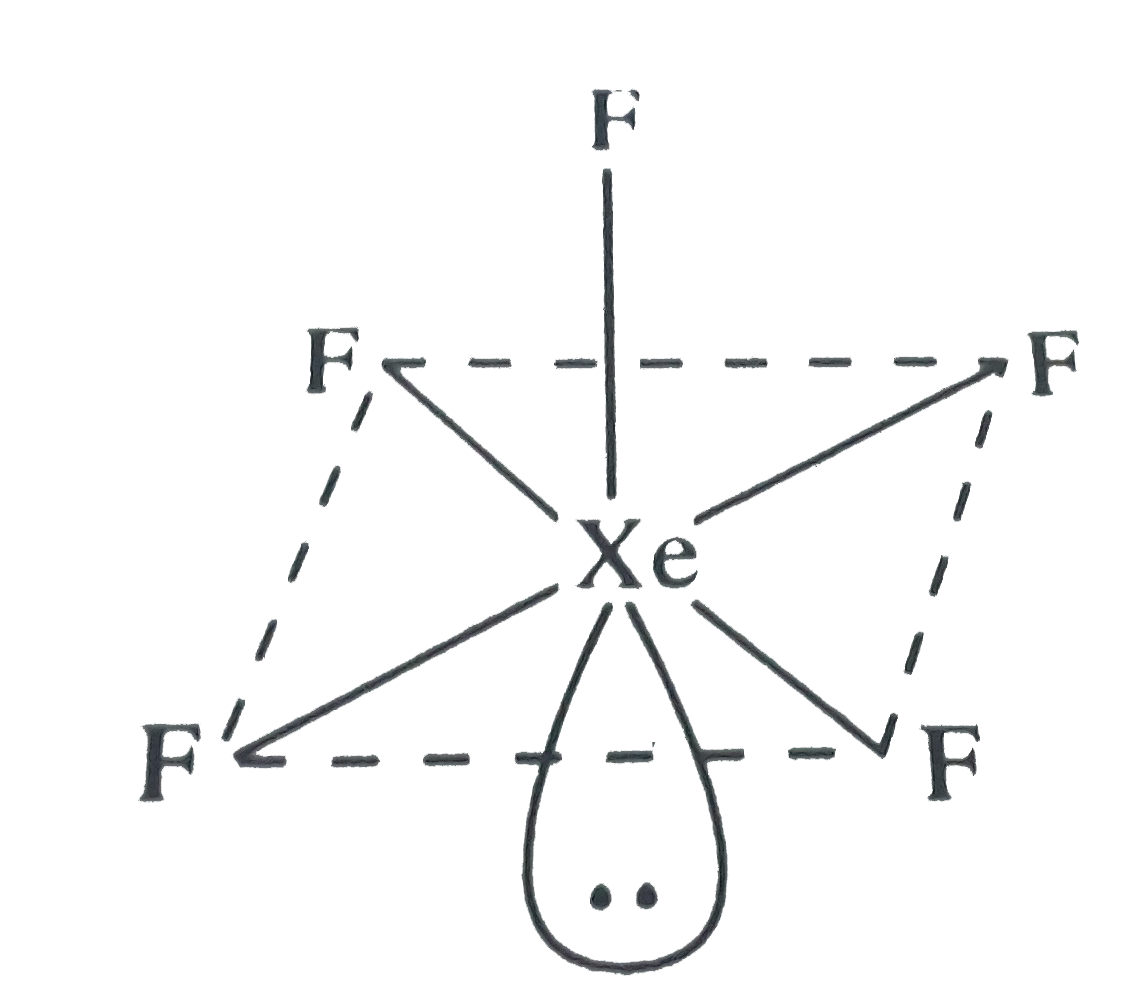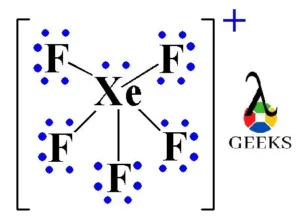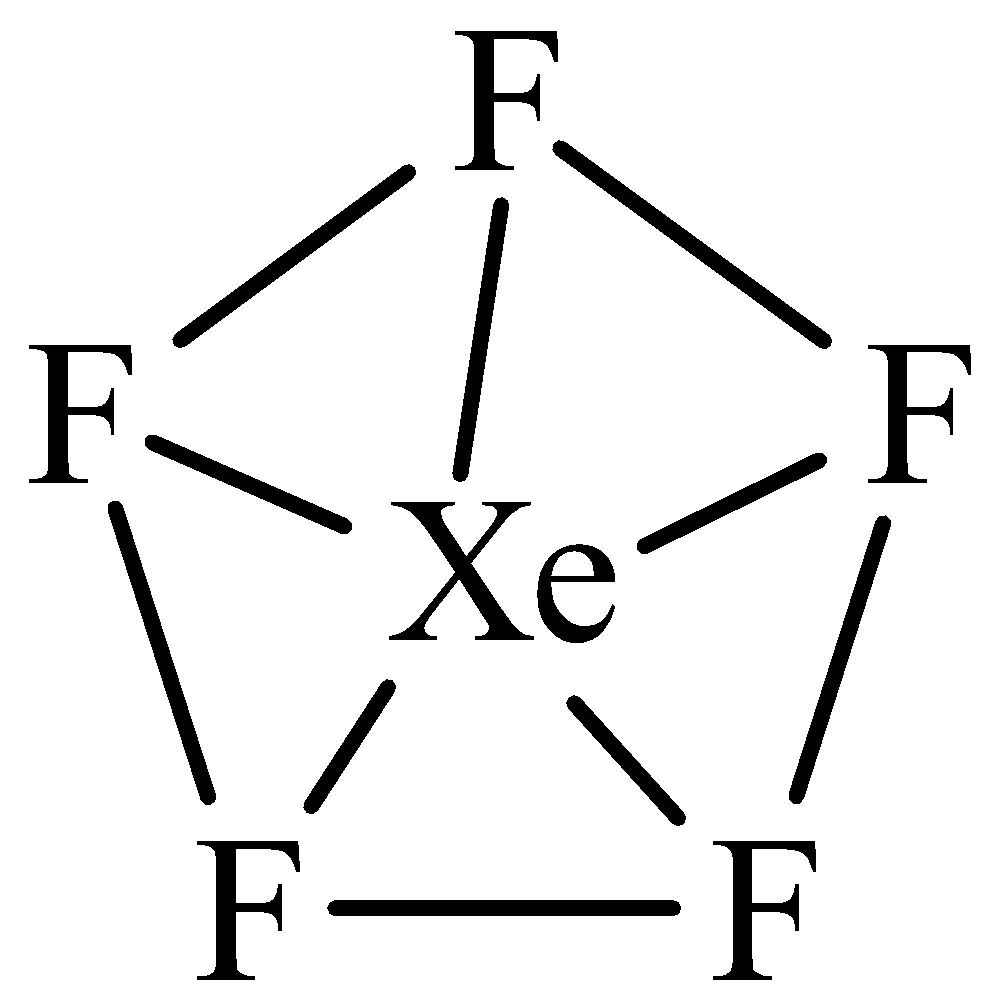
The hybridization and shape of $Xe{F_5}^ - $ is.A) $s{p^3}$, tetrahedron.B) $s{p^3}$, trigonal pyramidal.C) $s{p^3}{d^2}$, trigonal bipyramidal.D) $s{p^3}{d^3}$, pentagonal planar.
55. The sum of number of d orbitals whose lobes are available along axis and are involved in the hybridisation of Central atom of XeF5 and XeF+

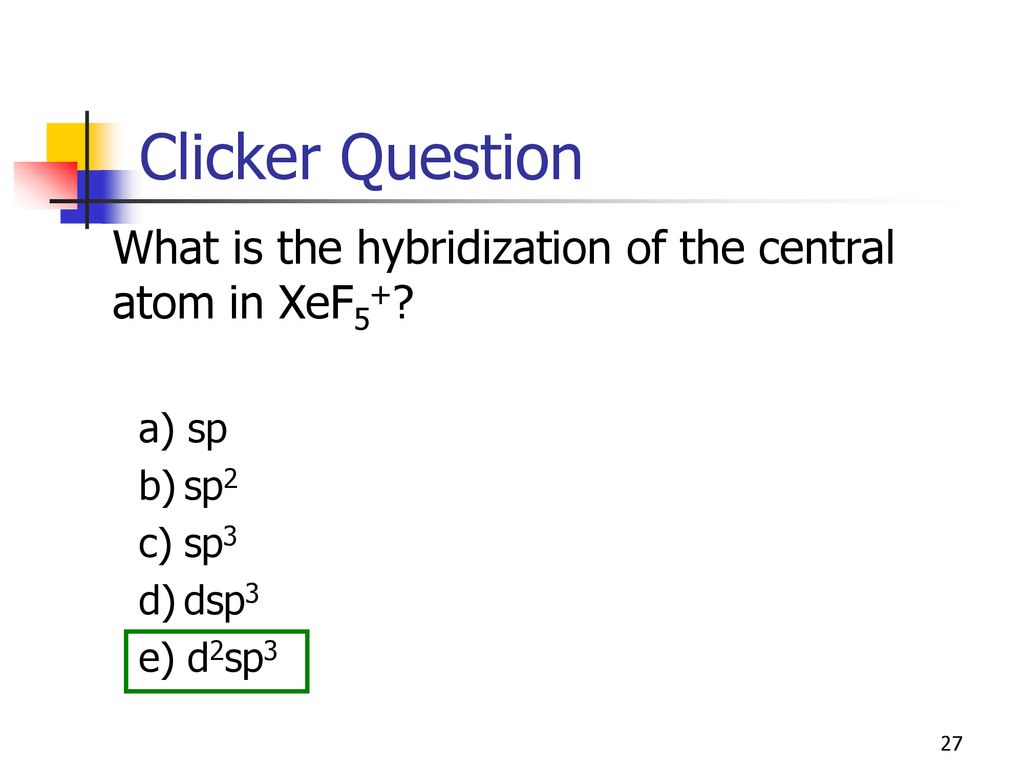
SOLVED: The hybridization of the central atom in xeF(5)^(+)is: sp^(3)d^(2) sp sp^(3) sp^(2) sp^(3)d The hybridization of the central atom in XeF5+ is: sp3d2 sp 0000 sp3 sp2 sp3d

Heats of Formation of XeF3+, XeF3−, XeF5+, XeF7+, XeF7−, and XeF8 from High Level Electronic Structure Calculations | Inorganic Chemistry

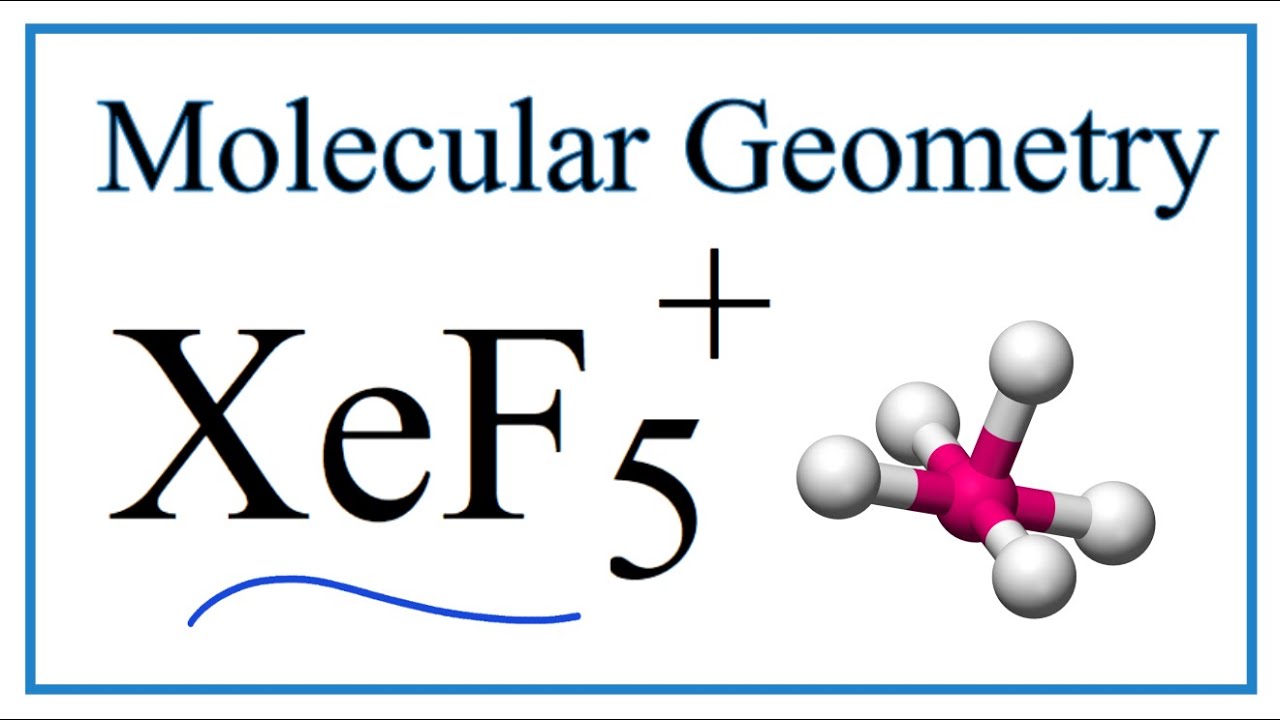
XeF5+ Lewis Structure: How to Draw the Lewis Structure for XeF5+ (Xenon pentafluoride cation) - YouTube
Why does Xenon Hexafluoride exist as [XeF₅]⁺ [F]¯ and not [XeF₅]⁺ [XeF₂]¯ in the solid state? - Quora
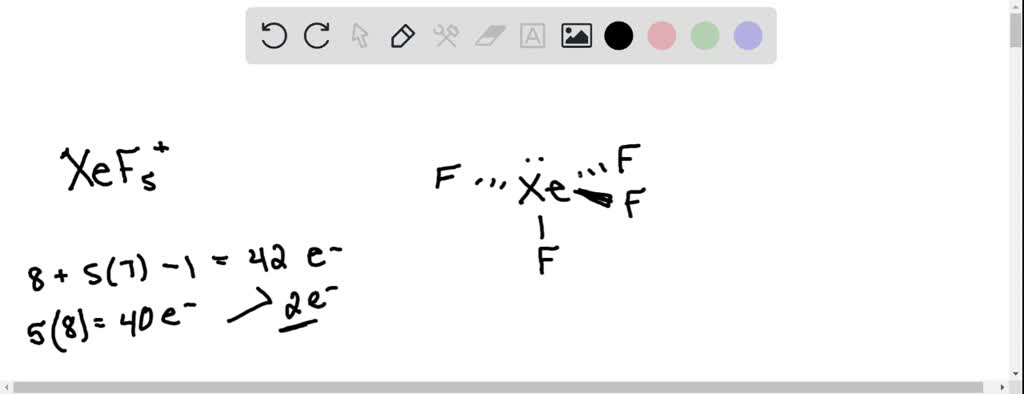
SOLVED: What is the Lewis dot structure for XeF5(+)? What is it's molecular shape? Polar or non polar? What is the hybridization of the central atom?

XeF5+ Lewis Structure: How to Draw the Lewis Structure for XeF5+ (Xenon pentafluoride cation) - YouTube

![The shape/structure of [XeF5]– and XeO3F2, respectively, are: The shape/structure of [XeF5]– and XeO3F2, respectively, are:](https://byjus-answer-creation.s3.amazonaws.com/uploads/8399Chemistry_62b6e470fe666010d3ec1df0b.jpg_img_upload_solution_2022-07-28%2012:54:00.369186.png)




![ANSWERED] In XeF5 molecule the lone pair electrons of Xe occupies - Kunduz ANSWERED] In XeF5 molecule the lone pair electrons of Xe occupies - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210912051139049283-3626449.jpg)


.jpg)